Difference between revisions of "Chalk"
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:50.727-SC26556.jpg|thumb|]] | + | [[File:50.727-SC26556.jpg|thumb|by Charles Daubigny<br>MFA# 50.727]] |
== Description == | == Description == | ||
| − | + | [[File:53.2441-SC25964.jpg|thumb|'Whaling' by Coleman<br>MFA# 53.2441]] | |
A soft, porous white mineral composed of fine-grain [[limestone]]. Also called whiting, pure varieties of chalk contain up to 99 percent [[calcium carbonate]] as the mineral [[calcite]]. Chalk was formed in the Cretaceous period and occurs naturally in thick beds in many parts of the world, such as the chalk cliffs along the English Channel. Chalk beds are collections of the shells of such tiny marine organisms as foraminifera, coccoliths, diatoms, and rhabdoliths. Ground chalk has been used as a pigment since ancient times. When mixed with [[animal glue|glue]], it was the most common ground for northern European paintings from medieval times well into the 18th century. Chalk was also commonly used for making [[lime]], [[portland cement]], [[putty]], and polishing powders. Industrially, it is still used as a filler, extender, or pigment in a wide variety of materials, including ceramics, putty, cosmetics, crayons, plastics, rubber, paper, paints, and [[linoleum]]. Chalk is stable and inert, but has poor covering power in oil. It helps control gloss, add texture, absorb oil, and provide plasticity. Chalk is made synthetically by precipitating fine particles of calcium carbonate. | A soft, porous white mineral composed of fine-grain [[limestone]]. Also called whiting, pure varieties of chalk contain up to 99 percent [[calcium carbonate]] as the mineral [[calcite]]. Chalk was formed in the Cretaceous period and occurs naturally in thick beds in many parts of the world, such as the chalk cliffs along the English Channel. Chalk beds are collections of the shells of such tiny marine organisms as foraminifera, coccoliths, diatoms, and rhabdoliths. Ground chalk has been used as a pigment since ancient times. When mixed with [[animal glue|glue]], it was the most common ground for northern European paintings from medieval times well into the 18th century. Chalk was also commonly used for making [[lime]], [[portland cement]], [[putty]], and polishing powders. Industrially, it is still used as a filler, extender, or pigment in a wide variety of materials, including ceramics, putty, cosmetics, crayons, plastics, rubber, paper, paints, and [[linoleum]]. Chalk is stable and inert, but has poor covering power in oil. It helps control gloss, add texture, absorb oil, and provide plasticity. Chalk is made synthetically by precipitating fine particles of calcium carbonate. | ||
| − | Note: [[Blackboard chalk]] is made from [[gypsum]] and tailors chalk is made from [[talc | + | Note: [[Blackboard chalk]] is made from [[gypsum]] and tailors chalk is made from [[talc]] |
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 12: | Line 10: | ||
calcium carbonate; Pigment White 18; CI 77220; whiting; Kreide (Deut.); craie (Fr.); calcare (It.); creta (Esp., It.); calcita (Esp.); tiza (Esp.); kimolia (Gr.); krijt (Ned.); kreda (Pol.); kalk (Sven.); cré (Port.); English white; Paris white; gilder's whiting; Champagne chalk; calcite; limestone; marble white; | calcium carbonate; Pigment White 18; CI 77220; whiting; Kreide (Deut.); craie (Fr.); calcare (It.); creta (Esp., It.); calcita (Esp.); tiza (Esp.); kimolia (Gr.); krijt (Ned.); kreda (Pol.); kalk (Sven.); cré (Port.); English white; Paris white; gilder's whiting; Champagne chalk; calcite; limestone; marble white; | ||
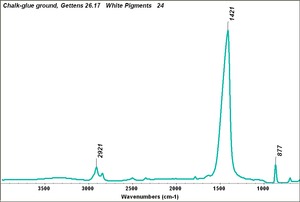
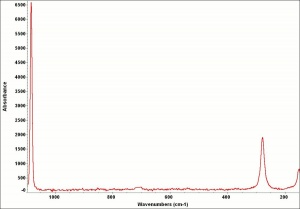
| − | [[[SliderGallery rightalign|Chalk-glue.TIF~FTIR (MFA)|ChalkUCL.jpg~Raman (UCL)]]] | + | [[[SliderGallery rightalign|Chalk-glue.TIF~FTIR (MFA)|ChalkUCL.jpg~Raman (UCL)|Calcite-XRF.png~XRF (MFA)]]] |
| − | + | * Composition = CaCO3 | |
| − | == | + | * Mohs Hardness = 3.0 |
| − | + | * Density = 1.8 - 2.7 g/ml | |
| − | Reacts with acids to evolve carbon dioxide. | + | * Refractive Index = e =1.486, w =1.64 -1.66 |
| − | + | * Reacts with acids to evolve carbon dioxide. | |
| − | High birefringence | + | * High birefringence with strong interference colors |
| − | + | * Microscopically, coccoliths may be seen at about 500x in natural chalk. | |
| − | Microscopically, coccoliths may be seen at about 500x in natural chalk. Precipitated chalk has fine, uniform particles | + | * Precipitated chalk has fine, uniform particles. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 48: | Line 25: | ||
[[media:download_file_525.pdf|Characteristics of Common White Pigments]] | [[media:download_file_525.pdf|Characteristics of Common White Pigments]] | ||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 60: | Line 35: | ||
</gallery> | </gallery> | ||
| − | + | == Resources Checked for Data in Record == | |
| − | == | + | * R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", ''Artists Pigments'', Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993. |
| − | |||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: e =1.486, w =1.64 -1.66 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: e =1.486, w =1.64 -1.66 | ||
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Chalk. Accessed May 25, 2003. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Chalk. | + | * Wikipedia: [https://en.wikipedia.org/wiki/Chalk Chalk] (Accessed Sept 2 2005 and March 2025) |
| − | |||
| − | * Wikipedia | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Chalk" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Chalk" | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 2.70 and ref. index = 1.510; 1.645 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 2.70 and ref. index = 1.510; 1.645 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 181 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 181 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
* M. Doerner, ''The Materials of the Artist'', Harcourt, Brace & Co., 1934 | * M. Doerner, ''The Materials of the Artist'', Harcourt, Brace & Co., 1934 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* R.M.Organ, ''Design for Scientific Conservation of Antiquities'', Smithsonian Institution, Washington DC, 1968 | * R.M.Organ, ''Design for Scientific Conservation of Antiquities'', Smithsonian Institution, Washington DC, 1968 | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 1.66; 1.44 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 1.66; 1.44 | ||
| − | |||
* Teri Hensick, contributed information, 1998 | * Teri Hensick, contributed information, 1998 | ||
| − | |||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| − | |||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | + | * Website: http://webexhibits.org/pigments/indiv/overview/chalk.html e =1.486, w =1.64 -1.66 | |
| − | * Website | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.9-2.8 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.9-2.8 | ||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:49, 26 April 2025
Description
A soft, porous white mineral composed of fine-grain Limestone. Also called whiting, pure varieties of chalk contain up to 99 percent Calcium carbonate as the mineral Calcite. Chalk was formed in the Cretaceous period and occurs naturally in thick beds in many parts of the world, such as the chalk cliffs along the English Channel. Chalk beds are collections of the shells of such tiny marine organisms as foraminifera, coccoliths, diatoms, and rhabdoliths. Ground chalk has been used as a pigment since ancient times. When mixed with glue, it was the most common ground for northern European paintings from medieval times well into the 18th century. Chalk was also commonly used for making Lime, Portland cement, Putty, and polishing powders. Industrially, it is still used as a filler, extender, or pigment in a wide variety of materials, including ceramics, putty, cosmetics, crayons, plastics, rubber, paper, paints, and Linoleum. Chalk is stable and inert, but has poor covering power in oil. It helps control gloss, add texture, absorb oil, and provide plasticity. Chalk is made synthetically by precipitating fine particles of calcium carbonate.
Note: Blackboard chalk is made from Gypsum and tailors chalk is made from Talc
Synonyms and Related Terms
calcium carbonate; Pigment White 18; CI 77220; whiting; Kreide (Deut.); craie (Fr.); calcare (It.); creta (Esp., It.); calcita (Esp.); tiza (Esp.); kimolia (Gr.); krijt (Ned.); kreda (Pol.); kalk (Sven.); cré (Port.); English white; Paris white; gilder's whiting; Champagne chalk; calcite; limestone; marble white;
- Composition = CaCO3
- Mohs Hardness = 3.0
- Density = 1.8 - 2.7 g/ml
- Refractive Index = e =1.486, w =1.64 -1.66
- Reacts with acids to evolve carbon dioxide.
- High birefringence with strong interference colors
- Microscopically, coccoliths may be seen at about 500x in natural chalk.
- Precipitated chalk has fine, uniform particles.
Comparisons
Properties of Common Abrasives
Characteristics of Common White Pigments
Additional Images
Resources Checked for Data in Record
- R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", Artists Pigments, Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive index: e =1.486, w =1.64 -1.66
- Encyclopedia Britannica, http://www.britannica.com Comment: Chalk. Accessed May 25, 2003.
- Wikipedia: Chalk (Accessed Sept 2 2005 and March 2025)
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Chalk"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 2.70 and ref. index = 1.510; 1.645
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 181
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- M. Doerner, The Materials of the Artist, Harcourt, Brace & Co., 1934
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- R.M.Organ, Design for Scientific Conservation of Antiquities, Smithsonian Institution, Washington DC, 1968
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 1.66; 1.44
- Teri Hensick, contributed information, 1998
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website: http://webexhibits.org/pigments/indiv/overview/chalk.html e =1.486, w =1.64 -1.66
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.9-2.8