Difference between revisions of "Desiccant"
Jump to navigation
Jump to search

| (19 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
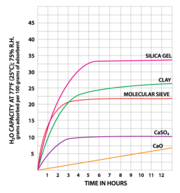
| − | [[File:Dessicant capacity chart.png|thumb|Desiccant Capacity chart (hours) | + | [[File:Dessicant capacity chart.png|thumb|Desiccant Capacity chart (hours); [https://www.sorbentsystems.com/desiccants_charts.html Sorbent Systems] ]] |
| − | [[File:Desicant capaity chart RH.png|thumb|Desiccant Capacity chart (RH) | + | [[File:Desicant capaity chart RH.png|thumb|Desiccant Capacity chart (RH); [https://www.sorbentsystems.com/desiccants_charts.html Sorbent Systems] ]] |
== Description == | == Description == | ||
| − | A hygroscopic substance that will remove water vapor from the air. Desiccants are used as drying agents. Some desiccants are chemically inert | + | A hygroscopic substance that will remove water vapor from the air. Desiccants are used as drying agents. Some desiccants are chemically inert, which allows the to be dried in an oven and reused, such as silica gel. Other desiccants react with water; these require special handling techniques. |
| − | Examples are [[activated carbon]], [[aerogel]], [[activated alumina]], [[calcium chloride]], [[calcium oxide]], [[calcium sulfate]], [[clay]], [[molecular sieve | + | Examples are [[activated carbon]], [[aerogel]], [[activated alumina]], [[calcium chloride]], [[calcium oxide]], [[calcium sulfate]], [[clay]], [[fumed silica]], [[molecular sieve]], [[potassium carbonate]], [[potassium hydroxide]], [[rice]], [[silica gel]], [[sodium]], [[sodium chlorate]], [[sodium chloride]], [[sodium hydroxide]], [[sodium sulfate]], [[sucrose]] and [[zinc chloride]]. |
| + | |||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Sorbent | ||
| + | ! Description | ||
| + | ! Risks | ||
| + | ! Adsorption Capacity | ||
| + | ! Regeneration conditions | ||
| + | ! Usage | ||
| + | |- | ||
| + | | [[Activated carbon]] | ||
| + | | A form of carbon containing small, low-volume pores that produce a high surface area to volume of material | ||
| + | | Non-toxic, non-corrosive; dust may cause irritation | ||
| + | | 15-25% | ||
| + | | | ||
| + | | Purification of air (odors, pollutants, moisture) and liquids; filtration; spill clean-up; chromatography | ||
| + | |- | ||
| + | | [[Activated alumina]] | ||
| + | | Dehydroxylated aluminum hydroxide producing a form of Al2O3 that is highly porous with high surface area; similar to silica gel but with lower capacity; preferentially adsorbs polar molecules | ||
| + | | Non-toxic; non-corrosive; dust may cause irritation | ||
| + | | 7.50% | ||
| + | | 175-315 C | ||
| + | | Purification of air (odors, pollutants, moisture) and liquids (heavy metals); filtration; selective adsorption in chemical production processes | ||
| + | |- | ||
| + | | [[Clay]] (montmorillonite or bentonite) | ||
| + | | Crystalline aluminosilicate with uniform network of pores; large surface area; selective absorption; chemically stable; does not release water easily | ||
| + | | Non toxic; recyclable when it does not contain toxins | ||
| + | | 15%-25% | ||
| + | | May release moisture at temperatures below 50C | ||
| + | | Readily absorbs most liquids and heavy metals; commonly used outdoors and in mixtures; cat litter | ||
| + | |- | ||
| + | | [[Calcium chloride]] | ||
| + | | Hygroscopic crystalline salt producing an exothermic reaction with water; depresses the freezing point of water | ||
| + | | Corrosive; use PPE for handling; | ||
| + | | | ||
| + | | -5-90 C | ||
| + | | Very high moisture absorption; often used in mixtures with clay; effective at high temperatures; de-icer | ||
| + | |- | ||
| + | | [[Calcium oxide]] (quicklime) | ||
| + | | Hygroscopic white powder; adsorption occurs slowly with swelling and heat production | ||
| + | | Caustic; use PPE for handling | ||
| + | | 28% | ||
| + | | | ||
| + | | Very high moisture absorption | ||
| + | |- | ||
| + | | [[Calcium sulfate, anhydrous|Calcium sulfate]] (anhydrite) | ||
| + | | Dehydrated gypsum; primarily used in labs; chemically stable; does not release water easily as it alters to form gypsum | ||
| + | | Non-corrosive; non-toxic and recyclable except when contains indicators | ||
| + | | 5-10% | ||
| + | | Limited regeneration; 175+ C | ||
| + | | Selectively absorbs water from air and liquids; gypsum is used in drywall because it forms anhydrite when burn, thus releasing water | ||
| + | |- | ||
| + | | [[Fumed silica]] | ||
| + | | A powder consisting of microscopic droplets of amorphous silica fused into three-dimensional particles with high surface structure and low bulk density; chemically stable. | ||
| + | | Non-corrosive; non-toxic | ||
| + | | 6% | ||
| + | | Not readily regenerated | ||
| + | | Widely used as thickener and filler in food storage and commercial products | ||
| + | |- | ||
| + | | [[Molecular sieve]] (synthetic Zeolite) | ||
| + | | Crystalline aluminosilicate with uniform network of pores; large surface area; selective absorption; chemically stable; does not release water easily | ||
| + | | Non-toxic; recyclable | ||
| + | | 10%-25% | ||
| + | | 230-330 C | ||
| + | | Affinity for water but absorbs most VOCs (limited by molecule size vs. pore size); can be used at high temps | ||
| + | |- | ||
| + | | [[Silica gel]] | ||
| + | | Partially dehydrated, amorphous form of silicic acid with interconnected micro pores producing very high surface structure; physical absorption; chemically stable; high mechanical strength; does not swell | ||
| + | | Non-corrosive; non-toxic except when contains indicators | ||
| + | | 10%-27%; most efficient below 25c | ||
| + | | 120C | ||
| + | | Widely used; food storage and commercial products; controlled humidity environments | ||
| + | |} | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
drier; drying agent; dessicant (sp); sorbent; scavenger | drier; drying agent; dessicant (sp); sorbent; scavenger | ||
| + | |||
| + | Commercial Products: | ||
| + | * Art Preservation Services: Arten Gel (silica gel for use from 0-45 %RH), Rhapid Gel (silica gel for use above 40%RH) | ||
| + | * Clariant: Desi Pak (bentonite clay); Sorb-It (silica gel); Tri-Sorb (molecular sieve) | ||
| + | * Uline: Container-Dri (CaCl) | ||
| + | * McMaster Carr: Silica gel (indicating and non-indicting); Clay; CaSO4; Aluminosilicate (for low humidities); Starch/CaCl (for high humidities) | ||
| + | * Sorbent Systems: StayDry paks (indicating silica gel or non-indicting silica gel or clay or activated carbon or custom blends); DriBox (Polycarbonate boxes with blue silica gel or orange silica gel or molecular sieves) | ||
| + | |||
| + | ==Usage Calculators== | ||
| + | For [[Silica gel, commercial|Silica Gel]]: | ||
| + | * Art Preservation Services: [https://www.apsnyc.com/demistfying-silica-gel Silica Gel Recommendations] | ||
| + | * Small Corp: [www.smallcorp.com/silica-gel-calculator Silica Gel Calculator] | ||
| + | * Gaylord: [www.gaylord.com/resources/silica-gel-calculators Silica Gel Calculator] | ||
==Resources and Citations== | ==Resources and Citations== | ||
| + | * AGM: [https://www.agmcontainer.com/blog/desiccant/selecting-desiccant/ Selecting the right desiccant] | ||
* Sorbent Systems: [https://www.sorbentsystems.com/desiccants_charts.html Desiccant Charts] | * Sorbent Systems: [https://www.sorbentsystems.com/desiccants_charts.html Desiccant Charts] | ||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | ||
| Line 19: | Line 107: | ||
| − | + | [[Category:Materials database]][[Category:MWG]][[Category:Climate/Environment]][[Category:Comparisons]] | |
| − | [[Category:Materials database]] | ||
Latest revision as of 07:01, 5 December 2023

Desiccant Capacity chart (hours); Sorbent Systems

Desiccant Capacity chart (RH); Sorbent Systems
Description
A hygroscopic substance that will remove water vapor from the air. Desiccants are used as drying agents. Some desiccants are chemically inert, which allows the to be dried in an oven and reused, such as silica gel. Other desiccants react with water; these require special handling techniques.
Examples are Activated carbon, Aerogel, Activated alumina, Calcium chloride, Calcium oxide, Calcium sulfate, Clay, Fumed silica, Molecular sieve, Potassium carbonate, Potassium hydroxide, Rice, Silica gel, Sodium, Sodium chlorate, Sodium chloride, Sodium hydroxide, Sodium sulfate, Sucrose and Zinc chloride.
| Sorbent | Description | Risks | Adsorption Capacity | Regeneration conditions | Usage |
|---|---|---|---|---|---|
| Activated carbon | A form of carbon containing small, low-volume pores that produce a high surface area to volume of material | Non-toxic, non-corrosive; dust may cause irritation | 15-25% | Purification of air (odors, pollutants, moisture) and liquids; filtration; spill clean-up; chromatography | |
| Activated alumina | Dehydroxylated aluminum hydroxide producing a form of Al2O3 that is highly porous with high surface area; similar to silica gel but with lower capacity; preferentially adsorbs polar molecules | Non-toxic; non-corrosive; dust may cause irritation | 7.50% | 175-315 C | Purification of air (odors, pollutants, moisture) and liquids (heavy metals); filtration; selective adsorption in chemical production processes |
| Clay (montmorillonite or bentonite) | Crystalline aluminosilicate with uniform network of pores; large surface area; selective absorption; chemically stable; does not release water easily | Non toxic; recyclable when it does not contain toxins | 15%-25% | May release moisture at temperatures below 50C | Readily absorbs most liquids and heavy metals; commonly used outdoors and in mixtures; cat litter |
| Calcium chloride | Hygroscopic crystalline salt producing an exothermic reaction with water; depresses the freezing point of water | Corrosive; use PPE for handling; | -5-90 C | Very high moisture absorption; often used in mixtures with clay; effective at high temperatures; de-icer | |
| Calcium oxide (quicklime) | Hygroscopic white powder; adsorption occurs slowly with swelling and heat production | Caustic; use PPE for handling | 28% | Very high moisture absorption | |
| Calcium sulfate (anhydrite) | Dehydrated gypsum; primarily used in labs; chemically stable; does not release water easily as it alters to form gypsum | Non-corrosive; non-toxic and recyclable except when contains indicators | 5-10% | Limited regeneration; 175+ C | Selectively absorbs water from air and liquids; gypsum is used in drywall because it forms anhydrite when burn, thus releasing water |
| Fumed silica | A powder consisting of microscopic droplets of amorphous silica fused into three-dimensional particles with high surface structure and low bulk density; chemically stable. | Non-corrosive; non-toxic | 6% | Not readily regenerated | Widely used as thickener and filler in food storage and commercial products |
| Molecular sieve (synthetic Zeolite) | Crystalline aluminosilicate with uniform network of pores; large surface area; selective absorption; chemically stable; does not release water easily | Non-toxic; recyclable | 10%-25% | 230-330 C | Affinity for water but absorbs most VOCs (limited by molecule size vs. pore size); can be used at high temps |
| Silica gel | Partially dehydrated, amorphous form of silicic acid with interconnected micro pores producing very high surface structure; physical absorption; chemically stable; high mechanical strength; does not swell | Non-corrosive; non-toxic except when contains indicators | 10%-27%; most efficient below 25c | 120C | Widely used; food storage and commercial products; controlled humidity environments |
Synonyms and Related Terms
drier; drying agent; dessicant (sp); sorbent; scavenger
Commercial Products:
- Art Preservation Services: Arten Gel (silica gel for use from 0-45 %RH), Rhapid Gel (silica gel for use above 40%RH)
- Clariant: Desi Pak (bentonite clay); Sorb-It (silica gel); Tri-Sorb (molecular sieve)
- Uline: Container-Dri (CaCl)
- McMaster Carr: Silica gel (indicating and non-indicting); Clay; CaSO4; Aluminosilicate (for low humidities); Starch/CaCl (for high humidities)
- Sorbent Systems: StayDry paks (indicating silica gel or non-indicting silica gel or clay or activated carbon or custom blends); DriBox (Polycarbonate boxes with blue silica gel or orange silica gel or molecular sieves)
Usage Calculators
For Silica Gel:
- Art Preservation Services: Silica Gel Recommendations
- Small Corp: [www.smallcorp.com/silica-gel-calculator Silica Gel Calculator]
- Gaylord: [www.gaylord.com/resources/silica-gel-calculators Silica Gel Calculator]
Resources and Citations
- AGM: Selecting the right desiccant
- Sorbent Systems: Desiccant Charts
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998