Difference between revisions of "Agate"
| Line 4: | Line 4: | ||
A cryptocrystalline variety of [[silica|silica]], showing a variegated banded structure and waxy luster. Agate bands are due to the deposition of successive mineral layers from solution and may be either straight, wavy, or concentric. Agate is slightly harder than [[quartz|quartz]]. It has been gathered or mined since Neolithic times. Sources for agate include western Asia, India, Brazil, Uruguay, and Germany. [[Onyx|Onyx]] is a type of agate in which the bands are parallel. [[Sardonyx|Sardonyx]] is a variety of onyx that has alternating red and cream layers. [[Moss%20agate|Moss agates]] contain brown dendritic or fernlike patterns. Agates are used for [[gemstone|gemstones]], beads, amulets, mortars and pestles, burnishers for gilding, textile rollers, knife edges, and as ornamental stones. | A cryptocrystalline variety of [[silica|silica]], showing a variegated banded structure and waxy luster. Agate bands are due to the deposition of successive mineral layers from solution and may be either straight, wavy, or concentric. Agate is slightly harder than [[quartz|quartz]]. It has been gathered or mined since Neolithic times. Sources for agate include western Asia, India, Brazil, Uruguay, and Germany. [[Onyx|Onyx]] is a type of agate in which the bands are parallel. [[Sardonyx|Sardonyx]] is a variety of onyx that has alternating red and cream layers. [[Moss%20agate|Moss agates]] contain brown dendritic or fernlike patterns. Agates are used for [[gemstone|gemstones]], beads, amulets, mortars and pestles, burnishers for gilding, textile rollers, knife edges, and as ornamental stones. | ||
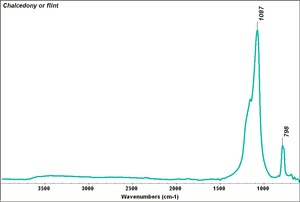
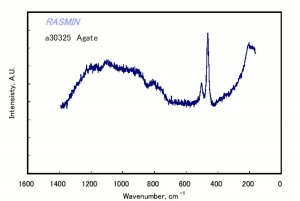
| − | [[[SliderGallery rightalign|agateRS.jpg~Raman]]] | + | [[[SliderGallery rightalign|Chalcedony or flint.TIF~FTIR (MFA)|agateRS.jpg~Raman (RASMIN)]]] |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 12: | Line 12: | ||
== Risks == | == Risks == | ||
| − | + | *May contain silica dust; chronic inhalation can cause silicosis. | |
| − | May contain silica dust; chronic inhalation can cause silicosis. | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 54: | Line 53: | ||
==Resources and Citations== | ==Resources and Citations== | ||
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
* Mineralogy Database: [http://www.webmineral.com/data/Quartz.shtml Quartz] | * Mineralogy Database: [http://www.webmineral.com/data/Quartz.shtml Quartz] | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density = 2.5-2.7 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density = 2.5-2.7 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.21 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.21 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* Robert Fournier, ''Illustrated Dictionary of Practical Pottery'', Chilton Book Company, Radnor, PA, 1992 | * Robert Fournier, ''Illustrated Dictionary of Practical Pottery'', Chilton Book Company, Radnor, PA, 1992 | ||
| − | |||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | |||
* Sue Fuller, ''Rocks and Minerals'', DK Publishing, Inc., New York City, 1995 | * Sue Fuller, ''Rocks and Minerals'', DK Publishing, Inc., New York City, 1995 | ||
| − | |||
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
| − | |||
* A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | ||
Revision as of 10:53, 19 December 2022
Description
A cryptocrystalline variety of Silica, showing a variegated banded structure and waxy luster. Agate bands are due to the deposition of successive mineral layers from solution and may be either straight, wavy, or concentric. Agate is slightly harder than Quartz. It has been gathered or mined since Neolithic times. Sources for agate include western Asia, India, Brazil, Uruguay, and Germany. Onyx is a type of agate in which the bands are parallel. Sardonyx is a variety of onyx that has alternating red and cream layers. Moss agates contain brown dendritic or fernlike patterns. Agates are used for gemstones, beads, amulets, mortars and pestles, burnishers for gilding, textile rollers, knife edges, and as ornamental stones.
Synonyms and Related Terms
Achat (Deut.); agat (Dan., Pol.); ágata (Esp., Port.); agathe (Fr.); agaat (Ned.)
Examples include: onyx; sardonyx
Risks
- May contain silica dust; chronic inhalation can cause silicosis.
Physical and Chemical Properties
- Fracture = conchoidal
- Luster = vitreous to waxy
- Streak = white to none
- Colors may include white, grey, light blue, orange, red, and black
- Fluorescence = generally inert
- Pleochroism = absent
| Composition | SiO2 |
|---|---|
| Mohs Hardness | 6.5-7.0 |
| Density | 2.59-2.61 g/ml |
| Refractive index | 1.535 - 1.539 |
| Birefringence | undetectable to 0.04 |
Comparisons
Properties of Common Gemstones
Additional Images
Resources and Citations
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- Mineralogy Database: Quartz
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density = 2.5-2.7
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.21
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Robert Fournier, Illustrated Dictionary of Practical Pottery, Chilton Book Company, Radnor, PA, 1992
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Sue Fuller, Rocks and Minerals, DK Publishing, Inc., New York City, 1995
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962