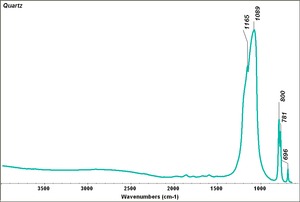
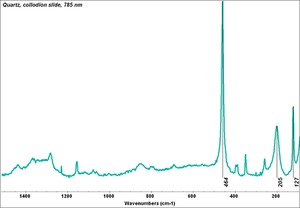
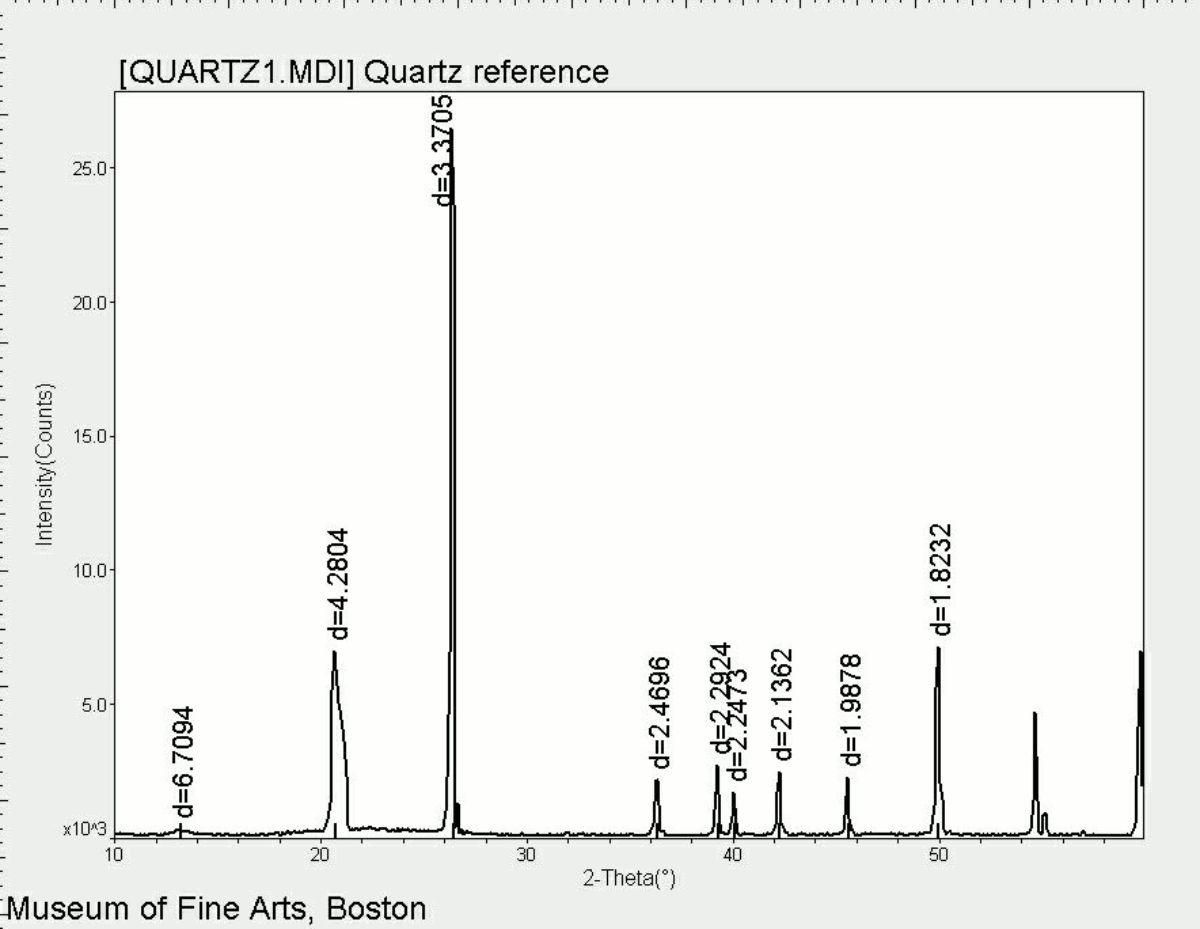
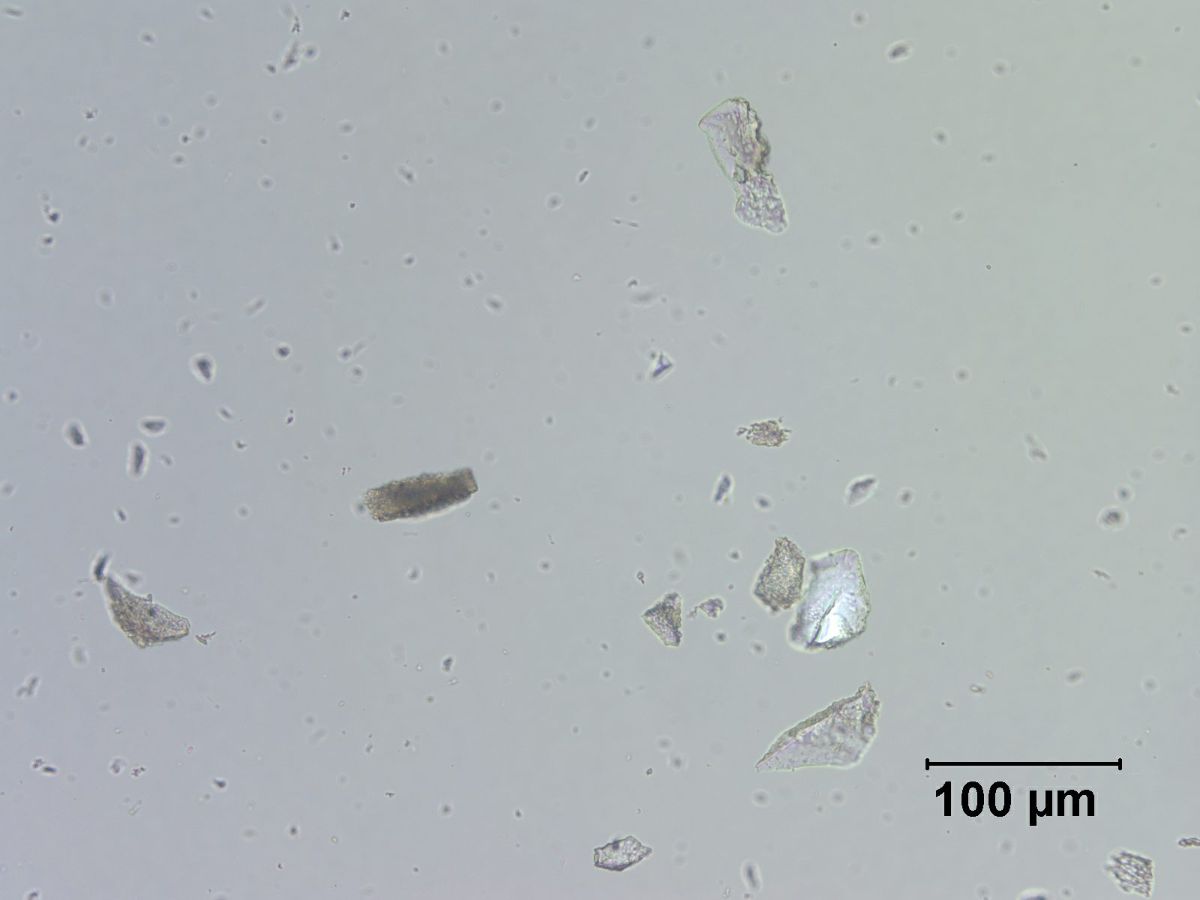
Quartz
Description
A hard, crystalline form of silicon dioxide. Quartz is one of the most common minerals in the earth's crust and occurs as grains (sand), masses (agate, bloodstone, chalcedony, jasper, carnelian, etc.) or crystals (rock crystal, amethyst, citrine, smoky quartz, rose quartz, etc.). Quartz usually crystallizes in hexagonal prisms or pyramids. It has been mined or gathered as a semiprecious stone since Paleolithic times. Quartz is a piezoelectric crystal, i.e. generates an electrical force when placed under pressure. Additionally, quartz crystals are used in polarized light microscopy because they can rotate the plane of polarized light. Some crystalline forms of quartz are used as gemstones, such as amethyst and citrine. Sand is the primary component in the manufacture of glass, and is an additive in porcelain, brick, cement, and mortar. Because of its hardness, quartz is also used as an abrasive in stonecutting, sandblasting, and glass grinding.
Synonyms and Related Terms
sand; agate; bloodstone; chalcedony; jasper; carnelian; sard; rock crystal (colorless); amethyst (purple); citrine (yellow); onyx; rose quartz (pink); smoky quartz (brown to black); yellow quartz; milky quartz (milk white); chrysoprase; kvarts (Dan., Nor., Sven.); Quarz (Deut.); cuarzo (Esp.); quartz (Fr.); quarzo (It.); kwarts (Ned.); kwarc (Pol.); quartzo (Port.)
Risks
- Noncombustible. Inhalation of fine particles may cause silicosis.
- US Silica Company: SDS
Physical and Chemical Properties
- Insoluble in acids except for hydrofluoric acid. Slightly soluble in alkalis
- Trigonal crystal system
- Low thermal expansion
- Fracture = conchoidal
- Luster = vitreous to greasy
- Streak = white
- Fluorescence = generally inert
- Microscopically, particles are transparent
- Crossed polars show low birefringence (0.009) and first order grays
- Pleochroism = very weak with different tones of body color
- Can be piezoeletric and/or triboluminescent
| Composition | SiO2 |
|---|---|
| CAS | 14808-60-7 |
| Mohs Hardness | 7.0 |
| Melting Point | 1713 C |
| Density | 2.65-2.66 g/ml |
| Molecular Weight | mol. wt. = 60.08 |
| Refractive Index | 1.544; 1.553 |
| Boiling Point | 2230 C |
Comparisons
Properties of Common Abrasives
Properties of Common Gemstones
Natural and Simulated Diamonds
Characteristics of Common White Pigments
Additional Images
Resources and Citations
- Mineralogy Database: Quartz
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- Wikipedia: Quartz (Accessed Dec 2022)
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 644
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- R.M.Organ, Design for Scientific Conservation of Antiquities, Smithsonian Institution, Washington DC, 1968
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- R.F.Symmes, T.T.Harding, Paul Taylor, Rocks, Fossils and Gems, DK Publishing, Inc., New York City, 1997
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "quartz" [Accessed December 4, 2001].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979