Difference between revisions of "Hematite"
| Line 6: | Line 6: | ||
[[File:1972.1172b-SC37892.jpg|thumb|Maya vessel<br>MFA# 1972.1172]] | [[File:1972.1172b-SC37892.jpg|thumb|Maya vessel<br>MFA# 1972.1172]] | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
iron (III) oxide; haematite (Br.); Hämatit (Deut.); Blutstein (Deut.); Eisenglanz (Deut.); Specularit (Deut.); Iserin (Deut.); Roteisenstein (Deut.); Roteisenerz (Deut.); hematites (Esp.); hématite (Fr.); hematiet (Ned.); hemtyt (Pol); hematite (Port.); iron sesquioxide; red iron ore; bloodstone; iron oxide red; red iron oxide; ferric oxide, red ocher; black diamond; iron rose; kidney ore; martite; paint ore; specularite | iron (III) oxide; haematite (Br.); Hämatit (Deut.); Blutstein (Deut.); Eisenglanz (Deut.); Specularit (Deut.); Iserin (Deut.); Roteisenstein (Deut.); Roteisenerz (Deut.); hematites (Esp.); hématite (Fr.); hematiet (Ned.); hemtyt (Pol); hematite (Port.); iron sesquioxide; red iron ore; bloodstone; iron oxide red; red iron oxide; ferric oxide, red ocher; black diamond; iron rose; kidney ore; martite; paint ore; specularite | ||
| − | |||
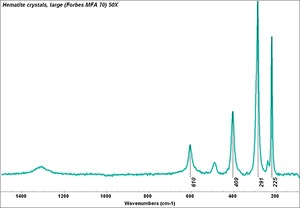
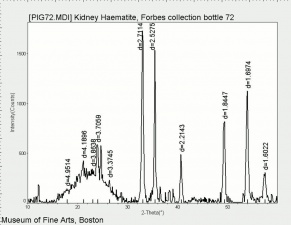
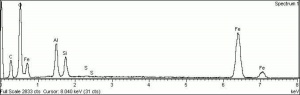
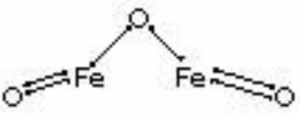
[[[SliderGallery rightalign|Hematite crystals, large (Forbes MFA 70) 50X resize.tif~Raman (MFA)|PIG72.jpg~XRD (MFA)|fkhaemsem.jpg~SEM (MFA)|fkhaemedsbw.jpg~EDS (MFA)|hematite.jpg~Chemical structure]]] | [[[SliderGallery rightalign|Hematite crystals, large (Forbes MFA 70) 50X resize.tif~Raman (MFA)|PIG72.jpg~XRD (MFA)|fkhaemsem.jpg~SEM (MFA)|fkhaemedsbw.jpg~EDS (MFA)|hematite.jpg~Chemical structure]]] | ||
| Line 20: | Line 18: | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| − | * | + | * Trigonal crystal system with thick granular or tabular crystals |
| − | * | + | * Cleavage = none |
| − | * Fracture = uneven to splintery | + | * Fracture = uneven to splintery |
| − | * Luster = metallic | + | * Luster = metallic |
| − | * Streak = dark red to cherry red | + | * Streak = dark red to cherry red |
| + | * Fluorescence = inert | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 48: | Line 47: | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 2.78; 3.01 | | 2.78; 3.01 | ||
| + | |- | ||
| + | ! scope="row"| Birefringence | ||
| + | | 0.280 (usually not detectable) | ||
|} | |} | ||
Latest revision as of 14:43, 24 January 2023
Description
A metallic black-gray or dark red mineral primarily composed of iron oxide. Hematite is commonly found throughout the world. The primary source for hematite is a sedimentary deposit in the Lake Superior district in North America. Other deposits include Brazil (Minas Gerais), Venezuela (Cerro Bolívar) and Canada (Labrador, Quebec). Hematite physically occurs in many forms: specular ore (steel gray color, shiny crystals); micaceous hematite (gray, scaly flakes), Red ocher (soft, fine-grain, red powder); kidney ore (massive, gray botryoidal form), and pencil ore (gray, fibrous crystals). Because hematite has a high Iron content (70%), it is primarily used for smelting iron. Hematite has been used since ancient times as a red pigment in paints and glazes. It was also used for seals, beads, and small carvings since the early 3rd millineum. Hematite is still used as a paint pigment. It is also used in Jewelers' rouge for polishing glass. Hematite can be used to produce the sparkle in Aventurine ceramic glazes.
Synonyms and Related Terms
iron (III) oxide; haematite (Br.); Hämatit (Deut.); Blutstein (Deut.); Eisenglanz (Deut.); Specularit (Deut.); Iserin (Deut.); Roteisenstein (Deut.); Roteisenerz (Deut.); hematites (Esp.); hématite (Fr.); hematiet (Ned.); hemtyt (Pol); hematite (Port.); iron sesquioxide; red iron ore; bloodstone; iron oxide red; red iron oxide; ferric oxide, red ocher; black diamond; iron rose; kidney ore; martite; paint ore; specularite
Risks
- Noncombustible. No significant hazards.
- Fisher Scientific: MSDS
Physical and Chemical Properties
- Trigonal crystal system with thick granular or tabular crystals
- Cleavage = none
- Fracture = uneven to splintery
- Luster = metallic
- Streak = dark red to cherry red
- Fluorescence = inert
| Composition | Fe2O3 |
|---|---|
| CAS | 1309-37-1; 1317-60-8 |
| Mohs Hardness | 5.5 - 6.5 |
| Melting Point | 2849 C |
| Density | 4.2-5.3 g/ml |
| Molecular Weight | mol. wt. = 159.6922 |
| Refractive Index | 2.78; 3.01 |
| Birefringence | 0.280 (usually not detectable) |
Comparisons
Properties of Common Abrasives
Additional Images
Resources and Citations
- Mineralogy Database: Hematite
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- Encyclopedia Britannica, http://www.britannica.com Comment: hematite" [Accessed December 11, 2001].
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: Hematite (Accessed Sept. 7, 2005 and Jn 2023)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- George Savage, Art and Antique Restorer's Handbook, Rockliff Publishing Corp, London, 1954
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=4.9-5.3