Difference between revisions of "Epoxy"
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A series of thermosetting polymers based on the chemical reactivity of an epoxide group. Epoxies, first patented in Switzerland in 1939 by Pierre Castan, were further developed in the 1940s and 50s. They are composed of a liquid that, when mixed with a catalyst, crosslinks to form a hard, strongly bound solid. The most commonly used epoxies are made with epichlorohydrin reacted with bisphenol A. Epoxies are typically dense, insoluble structures that are dimensionally stable and wear resistant. | + | A series of thermosetting polymers based on the chemical reactivity of an epoxide group. Epoxies, first patented in Switzerland in 1939 by Pierre Castan, were further developed in the 1940s and 50s. They are composed of a liquid that, when mixed with a catalyst, crosslinks to form a hard, strongly bound solid. The most commonly used epoxies are made with epichlorohydrin reacted with bisphenol A. Epoxies are typically dense, insoluble structures that are dimensionally stable and wear resistant. Exhibiting minimal shrinkage with cure, they are used as adhesives, molded products, and baked enamel surface coatings. Some commercial brands that contain epoxies are: [[Ablebond]], [[Araldite]], [[Epon resins]], [[Epo-Tek 301|Epo-tek]], [[Hxtal NYL-1|Hxtal]], [[Phillyseal R]] (formerly Pliacre) and [[UHU adhesives]]. |
[[File:1.18.05 06-Applying epoxy.jpg|thumb|Applying epoxy]] | [[File:1.18.05 06-Applying epoxy.jpg|thumb|Applying epoxy]] | ||
| Line 13: | Line 13: | ||
== Applications == | == Applications == | ||
| + | * Adhesive | ||
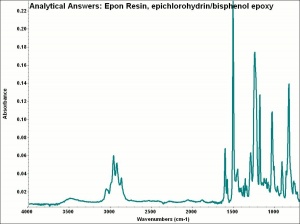
[[[SliderGallery rightalign|aaiEPON_epoxy.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiEPON_epoxy.jpg~FTIR]]] | ||
| − | == Risks == | + | == Personal Risks == |
| − | Hardeners are moderately toxic by ingestion, inhalation and skin contact. | + | Hardeners are moderately toxic by ingestion, inhalation and skin contact. |
| − | == | + | == Environmental Risks == |
| − | + | == Collection Risks == | |
| + | May form ammonia and hydrochloric acid upon degradation. | ||
| − | + | == Physical and Chemical Properties == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | = | + | * Burns with yellow flame; smells of phenol. |
| + | * Density = 1.1-1.4 | ||
| − | + | == Working Properties == | |
| − | + | Two-part system with a liquid and a catalyst. Curing time ranges by product from 5 minutes - a week | |
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 49: | Line 47: | ||
</gallery> | </gallery> | ||
| − | == | + | == Resources and Citations == |
| + | * J. Down, M.MacDonald, J.Te'treault, S.Williams, Adhesive Testing at the Canadian Conservation Institute-An Evaluation of Selected Poly(Vinyl acetate) and Acrylic Adhesives", ''Studies in Conservation'' 41:19-44, 1996. | ||
| + | |||
| + | * M.Savla, "Epoxy Resin Adhesives" in ''Handbook of Adhesives'', I.Skeist (ed.), Van Nostrand Reinhold, New York, 1977, p.434-445. | ||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | ||
| Line 68: | Line 69: | ||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | |||
| − | |||
* Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html | * Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html | ||
Revision as of 09:58, 2 July 2020
Description
A series of thermosetting polymers based on the chemical reactivity of an epoxide group. Epoxies, first patented in Switzerland in 1939 by Pierre Castan, were further developed in the 1940s and 50s. They are composed of a liquid that, when mixed with a catalyst, crosslinks to form a hard, strongly bound solid. The most commonly used epoxies are made with epichlorohydrin reacted with bisphenol A. Epoxies are typically dense, insoluble structures that are dimensionally stable and wear resistant. Exhibiting minimal shrinkage with cure, they are used as adhesives, molded products, and baked enamel surface coatings. Some commercial brands that contain epoxies are: Ablebond, Araldite, Epon resins, Epo-tek, Hxtal, Phillyseal R (formerly Pliacre) and UHU adhesives.
Synonyms and Related Terms
EP; epoxy resin; epoxide resin; polyepoxide; résine époxyde (Fr.); Epoxidharz (Deut.); resina epoxi (Esp.); poliepóxido (Esp., Port.); resina epossidica (It.); resina epoxídica (Port.); epoxi (Sven.)
Examples: Ablebond; Araldite [Huntsman ex Ciba-Geigy]; CM Bond; Epon® [Hexion]; Epotek; Hxtal; Phillyseal R (formerly Pliacre) [Philadelphia Resins]; UHU
Applications
- Adhesive
Personal Risks
Hardeners are moderately toxic by ingestion, inhalation and skin contact.
Environmental Risks
Collection Risks
May form ammonia and hydrochloric acid upon degradation.
Physical and Chemical Properties
- Burns with yellow flame; smells of phenol.
- Density = 1.1-1.4
Working Properties
Two-part system with a liquid and a catalyst. Curing time ranges by product from 5 minutes - a week
Comparisons
Physical Properties for Selected Thermoset Resins
General Characteristics of Polymers
Additional Images
Resources and Citations
- J. Down, M.MacDonald, J.Te'treault, S.Williams, Adhesive Testing at the Canadian Conservation Institute-An Evaluation of Selected Poly(Vinyl acetate) and Acrylic Adhesives", Studies in Conservation 41:19-44, 1996.
- M.Savla, "Epoxy Resin Adhesives" in Handbook of Adhesives, I.Skeist (ed.), Van Nostrand Reinhold, New York, 1977, p.434-445.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Epoxy (Accessed Feb. 10, 2006)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000