Difference between revisions of "Morin"
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 2: | Line 2: | ||
A colorant found in [[fustic|old fustic]], Cuba wood, yellow brazilwood, jakwood, and [[Osage%20orange|Osage orange tree]]. Morin crystallizes from [[ethyl%20alcohol|ethanol]] to form colorless needles. It turns an intense yellow in alkaline solutions, but will slowly turn brown with exposure to air. Morin is used as a dye for [[wool|wools]]: with [[chromium|chromium]] it gives an olive green, with [[aluminum|aluminum]] and [[tin|tin]], it is yellow, and with [[iron|iron]], morin is a deep olive brown. It is also used as a spot test reagent for salts of [[aluminum|aluminum]], [[beryllium|beryllium]], [[zinc|zinc]], [[gallium|gallium]], [[indium|indium]], and [[selenium|selenium]]. | A colorant found in [[fustic|old fustic]], Cuba wood, yellow brazilwood, jakwood, and [[Osage%20orange|Osage orange tree]]. Morin crystallizes from [[ethyl%20alcohol|ethanol]] to form colorless needles. It turns an intense yellow in alkaline solutions, but will slowly turn brown with exposure to air. Morin is used as a dye for [[wool|wools]]: with [[chromium|chromium]] it gives an olive green, with [[aluminum|aluminum]] and [[tin|tin]], it is yellow, and with [[iron|iron]], morin is a deep olive brown. It is also used as a spot test reagent for salts of [[aluminum|aluminum]], [[beryllium|beryllium]], [[zinc|zinc]], [[gallium|gallium]], [[indium|indium]], and [[selenium|selenium]]. | ||
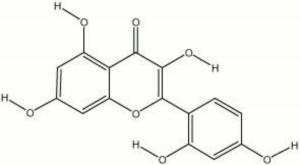
| − | + | [[[SliderGallery rightalign|morin.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
2',3,4,5,7-pentahydroxyflavone; Natural Yellow 8; Natural Yellow 11; CI 75660; morina (Esp., Port.); morin hydrate; moric acid | 2',3,4,5,7-pentahydroxyflavone; Natural Yellow 8; Natural Yellow 11; CI 75660; morina (Esp., Port.); morin hydrate; moric acid | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustible. | ||
| + | * Contact may cause irritation. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/03247.htm MSDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in ethanol, alkalis, acetic acid. Slightly soluble in water. | Soluble in ethanol, alkalis, acetic acid. Slightly soluble in water. | ||
| Line 24: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 285 | + | | 285 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 30: | Line 34: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 | * F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 | ||
Latest revision as of 08:37, 19 October 2022
Description
A colorant found in old fustic, Cuba wood, yellow brazilwood, jakwood, and Osage orange tree. Morin crystallizes from ethanol to form colorless needles. It turns an intense yellow in alkaline solutions, but will slowly turn brown with exposure to air. Morin is used as a dye for wools: with Chromium it gives an olive green, with Aluminum and Tin, it is yellow, and with Iron, morin is a deep olive brown. It is also used as a spot test reagent for salts of Aluminum, Beryllium, Zinc, Gallium, Indium, and Selenium.
Synonyms and Related Terms
2',3,4,5,7-pentahydroxyflavone; Natural Yellow 8; Natural Yellow 11; CI 75660; morina (Esp., Port.); morin hydrate; moric acid
Risks
- Combustible.
- Contact may cause irritation.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol, alkalis, acetic acid. Slightly soluble in water.
Soluble in sulfuric acid forming a solution with green fluorescence.
| Composition | C15H10O7-2H2O |
|---|---|
| CAS | 480-16-0 |
| Melting Point | 285 C |
| Molecular Weight | mol. wt. = 302.23 |
Resources and Citations
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876
- Judith Hofenk-de Graaff, Natural Dyestuffs: Origin, Chemical Constitution, Identification, Central Research Laboratory for Objects of Art and Science, Amsterdam, 1969
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6352