Difference between revisions of "Quartz"
| (12 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:19.1562-CR9463-d1.jpg|thumb|]] | + | [[File:19.1562-CR9463-d1.jpg|thumb|Diamond-shaped pallette<br>MFA# 19.1562]] |
== Description == | == Description == | ||
| − | + | [[File:24.884-CR9404-d1.jpg|thumb|Archer's Draw ring<br>MFA# 24.884]] | |
A hard, crystalline form of [[silica|silicon dioxide]]. Quartz is one of the most common minerals in the earth's crust and occurs as grains ([[sand]]), masses ([[agate]], [[bloodstone]], [[chalcedony]], [[jasper]], [[carnelian]], etc.) or crystals ([[rock crystal]], [[amethyst]], [[citrine]], [[smoky quartz]], [[rose quartz]], etc.). Quartz usually crystallizes in hexagonal prisms or pyramids. It has been mined or gathered as a semiprecious stone since Paleolithic times. Quartz is a piezoelectric crystal, i.e. generates an electrical force when placed under pressure. Additionally, quartz crystals are used in polarized light microscopy because they can rotate the plane of polarized light. Some crystalline forms of quartz are used as gemstones, such as amethyst and citrine. Sand is the primary component in the manufacture of glass, and is an additive in porcelain, brick, cement, and mortar. Because of its hardness, quartz is also used as an abrasive in stonecutting, sandblasting, and glass grinding. | A hard, crystalline form of [[silica|silicon dioxide]]. Quartz is one of the most common minerals in the earth's crust and occurs as grains ([[sand]]), masses ([[agate]], [[bloodstone]], [[chalcedony]], [[jasper]], [[carnelian]], etc.) or crystals ([[rock crystal]], [[amethyst]], [[citrine]], [[smoky quartz]], [[rose quartz]], etc.). Quartz usually crystallizes in hexagonal prisms or pyramids. It has been mined or gathered as a semiprecious stone since Paleolithic times. Quartz is a piezoelectric crystal, i.e. generates an electrical force when placed under pressure. Additionally, quartz crystals are used in polarized light microscopy because they can rotate the plane of polarized light. Some crystalline forms of quartz are used as gemstones, such as amethyst and citrine. Sand is the primary component in the manufacture of glass, and is an additive in porcelain, brick, cement, and mortar. Because of its hardness, quartz is also used as an abrasive in stonecutting, sandblasting, and glass grinding. | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
sand; agate; bloodstone; chalcedony; jasper; carnelian; sard; rock crystal (colorless); amethyst (purple); citrine (yellow); onyx; rose quartz (pink); smoky quartz (brown to black); yellow quartz; milky quartz (milk white); chrysoprase; kvarts (Dan., Nor., Sven.); Quarz (Deut.); cuarzo (Esp.); quartz (Fr.); quarzo (It.); kwarts (Ned.); kwarc (Pol.); quartzo (Port.) | sand; agate; bloodstone; chalcedony; jasper; carnelian; sard; rock crystal (colorless); amethyst (purple); citrine (yellow); onyx; rose quartz (pink); smoky quartz (brown to black); yellow quartz; milky quartz (milk white); chrysoprase; kvarts (Dan., Nor., Sven.); Quarz (Deut.); cuarzo (Esp.); quartz (Fr.); quarzo (It.); kwarts (Ned.); kwarc (Pol.); quartzo (Port.) | ||
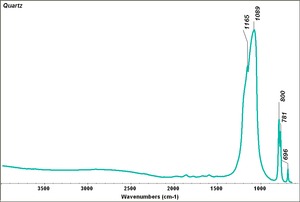
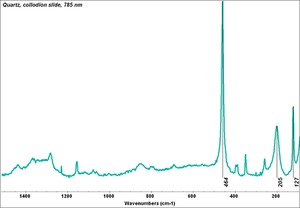
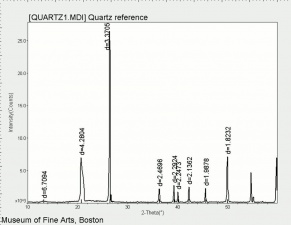
| − | [[[SliderGallery rightalign|Quartz | + | [[[SliderGallery rightalign|Quartz.TIF~FTIR (MFA)|Quartz, collodion slide, 785 nm copy.tif~Raman (MFA)|QUARTZ1.jpg~XRD (MFA)]]] |
| − | + | == Risks == | |
| − | == | ||
| − | |||
| − | |||
| − | + | * Noncombustible. Inhalation of fine particles may cause silicosis. | |
| + | * US Silica Company: [https://www.ussilica.com/sites/default/files/2019-05/Silica%20OSHA%20GHS%20SDS%20%288-17%29.pdf SDS] | ||
| + | == Physical and Chemical Properties == | ||
| − | Fracture = conchoidal | + | * Insoluble in acids except for hydrofluoric acid. Slightly soluble in alkalis |
| − | + | * Trigonal crystal system | |
| − | Microscopically, particles are transparent | + | * Low thermal expansion |
| + | * Fracture = conchoidal | ||
| + | * Luster = vitreous to greasy | ||
| + | * Streak = white | ||
| + | * Fluorescence = generally inert | ||
| + | * Microscopically, particles are transparent | ||
| + | * Crossed polars show low birefringence (0.009) and first order grays | ||
| + | * Pleochroism = very weak with different tones of body color | ||
| + | * Can be piezoeletric and/or triboluminescent | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 33: | Line 39: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 1713 | + | | 1713 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.65-2.66 | + | | 2.65-2.66 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 45: | Line 51: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 2230 | + | | 2230 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_209.pdf|Properties of Common Abrasives]] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[media: | + | [[media:download_file_414.pdf|Properties of Common Gemstones]] |
| + | [[media:download_file_446.pdf|Natural and Simulated Diamonds]] | ||
| + | [[media:download_file_512.pdf|Characteristics of Common White Pigments]] | ||
== Additional Images == | == Additional Images == | ||
| Line 80: | Line 74: | ||
</gallery> | </gallery> | ||
| + | ==Resources and Citations== | ||
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Quartz.shtml Quartz] | |
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
| + | * Wikipedia: [https://en.wikipedia.org/wiki/Quartz Quartz] (Accessed Dec 2022) | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 644 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 644 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* R.M.Organ, ''Design for Scientific Conservation of Antiquities'', Smithsonian Institution, Washington DC, 1968 | * R.M.Organ, ''Design for Scientific Conservation of Antiquities'', Smithsonian Institution, Washington DC, 1968 | ||
| − | |||
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* R.F.Symmes, T.T.Harding, Paul Taylor, ''Rocks, Fossils and Gems'', DK Publishing, Inc., New York City, 1997 | * R.F.Symmes, T.T.Harding, Paul Taylor, ''Rocks, Fossils and Gems'', DK Publishing, Inc., New York City, 1997 | ||
| − | |||
* A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "quartz" [Accessed December 4, 2001]. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "quartz" | ||
| − | |||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
Latest revision as of 11:37, 26 December 2022
Description
A hard, crystalline form of silicon dioxide. Quartz is one of the most common minerals in the earth's crust and occurs as grains (Sand), masses (Agate, Bloodstone, Chalcedony, Jasper, Carnelian, etc.) or crystals (Rock crystal, Amethyst, Citrine, Smoky quartz, Rose quartz, etc.). Quartz usually crystallizes in hexagonal prisms or pyramids. It has been mined or gathered as a semiprecious stone since Paleolithic times. Quartz is a piezoelectric crystal, i.e. generates an electrical force when placed under pressure. Additionally, quartz crystals are used in polarized light microscopy because they can rotate the plane of polarized light. Some crystalline forms of quartz are used as gemstones, such as amethyst and citrine. Sand is the primary component in the manufacture of glass, and is an additive in porcelain, brick, cement, and mortar. Because of its hardness, quartz is also used as an abrasive in stonecutting, sandblasting, and glass grinding.
Synonyms and Related Terms
sand; agate; bloodstone; chalcedony; jasper; carnelian; sard; rock crystal (colorless); amethyst (purple); citrine (yellow); onyx; rose quartz (pink); smoky quartz (brown to black); yellow quartz; milky quartz (milk white); chrysoprase; kvarts (Dan., Nor., Sven.); Quarz (Deut.); cuarzo (Esp.); quartz (Fr.); quarzo (It.); kwarts (Ned.); kwarc (Pol.); quartzo (Port.)
Risks
- Noncombustible. Inhalation of fine particles may cause silicosis.
- US Silica Company: SDS
Physical and Chemical Properties
- Insoluble in acids except for hydrofluoric acid. Slightly soluble in alkalis
- Trigonal crystal system
- Low thermal expansion
- Fracture = conchoidal
- Luster = vitreous to greasy
- Streak = white
- Fluorescence = generally inert
- Microscopically, particles are transparent
- Crossed polars show low birefringence (0.009) and first order grays
- Pleochroism = very weak with different tones of body color
- Can be piezoeletric and/or triboluminescent
| Composition | SiO2 |
|---|---|
| CAS | 14808-60-7 |
| Mohs Hardness | 7.0 |
| Melting Point | 1713 C |
| Density | 2.65-2.66 g/ml |
| Molecular Weight | mol. wt. = 60.08 |
| Refractive Index | 1.544; 1.553 |
| Boiling Point | 2230 C |
Comparisons
Properties of Common Abrasives
Properties of Common Gemstones
Natural and Simulated Diamonds
Characteristics of Common White Pigments
Additional Images
Resources and Citations
- Mineralogy Database: Quartz
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- Wikipedia: Quartz (Accessed Dec 2022)
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 644
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- R.M.Organ, Design for Scientific Conservation of Antiquities, Smithsonian Institution, Washington DC, 1968
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- R.F.Symmes, T.T.Harding, Paul Taylor, Rocks, Fossils and Gems, DK Publishing, Inc., New York City, 1997
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "quartz" [Accessed December 4, 2001].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979