Difference between revisions of "Lead white"
| (3 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
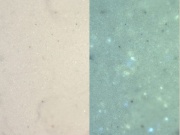
[[File:Leadwhite C100x.jpg|thumb|Lead white at 100x (visible light left; UV light right)]] | [[File:Leadwhite C100x.jpg|thumb|Lead white at 100x (visible light left; UV light right)]] | ||
[[File:4_White_lead_200X.jpg|thumb|Lead white at 200x]] | [[File:4_White_lead_200X.jpg|thumb|Lead white at 200x]] | ||
| − | A white pigment composed of [[lead carbonate, basic|basic lead carbonate]]. Lead white (lead hydroxycarbonate), contains about 70% lead carbonate and 30% lead hydroxide chemically combined. Basic lead carbonate occurs in nature as the mineral hydrocerussite, but the mineral form was not commonly used as a pigment; instead lead white has been prepared synthetically from Classical antiquity by exposing metallic [[lead]] to [[vinegar]] ([[acetic acid]]) | + | A white pigment composed of [[lead carbonate, basic|basic lead carbonate]]. Lead white (lead hydroxycarbonate), contains about 70% lead carbonate and 30% lead hydroxide chemically combined. Basic lead carbonate occurs in nature as the mineral hydrocerussite, but the mineral form was not commonly used as a pigment; instead lead white has been prepared synthetically from Classical antiquity by exposing metallic [[lead]] to [[vinegar]] ([[acetic acid]]) vapor. [[Lead acetate]] forms first, which then reacts with [[carbon dioxide]] from the atmosphere (or from another source). Lead white is a dense, opaque pigment that was mainly used in drying oils where it acts as a [[drier|siccative]]. It has also been found in [[egg tempera]], [[glue tempera]], and [[gum tempera]], but it was not considered suitable for [[buon fresco]] techniques. Lead white was the principal white pigment used in paintings and ceramic glazes from ancient times until the early 20th century when it was replaced by [[zinc white]] and [[titanium white]]. Unfortunately, lead white was still found in some exterior house paints and ceramic glazes till the middle of the mid 20th century. In the United States, its use in interior paints was restricted in the 1950s and prohibited in 1978. Lead white can yellow or blacken in the presence of sulfides or alkalis. Additionally, it can be oxidized by microorganisms to form brownish-black lead dioxide. [[Cremnitz white]] is a very white, dense type of white lead prepared from [[litharge]] and acetic acid. Occasionally the name lead white has also been used for [[lead sulfate, basic|basic lead sulfate]] and [[lead silicate|basic lead silicate]]. |
| − | |||
[[File:4_White_lead_200X_pol.jpg|thumb|Lead white at 200x, polarized light]] | [[File:4_White_lead_200X_pol.jpg|thumb|Lead white at 200x, polarized light]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 10: | Line 9: | ||
basic lead carbonate; Pigment White 1; CI 77597; hydrocerussite (mineral); plomo blanco (Esp.); céruse (Fr.); blanc de plomb (Fr.); blanc d'argent (Fr.); Bleiweiss (Deut.); Kremserweiss (Deut.); leyko toy molybdoy (Gr.); psimythio (Gr.); ydrokeroyssitis (Gr.)bianco di piombo (It.); biacca (It.); cerussa (It.); loodwit (Ned.); branco de chumbo (Port.); enpaku (Jap.); bai (Chin.); Flemish white; Vienna white; Berlin white; slate white; Roman white; flake white; Cremnitz white; white lead; London white; silver white; Krems white; Dutch white lead | basic lead carbonate; Pigment White 1; CI 77597; hydrocerussite (mineral); plomo blanco (Esp.); céruse (Fr.); blanc de plomb (Fr.); blanc d'argent (Fr.); Bleiweiss (Deut.); Kremserweiss (Deut.); leyko toy molybdoy (Gr.); psimythio (Gr.); ydrokeroyssitis (Gr.)bianco di piombo (It.); biacca (It.); cerussa (It.); loodwit (Ned.); branco de chumbo (Port.); enpaku (Jap.); bai (Chin.); Flemish white; Vienna white; Berlin white; slate white; Roman white; flake white; Cremnitz white; white lead; London white; silver white; Krems white; Dutch white lead | ||
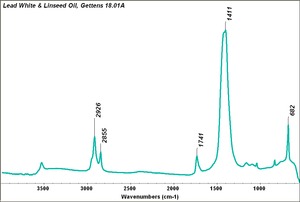
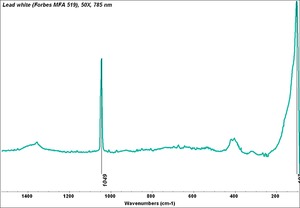
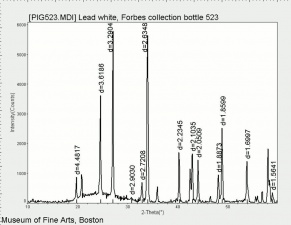
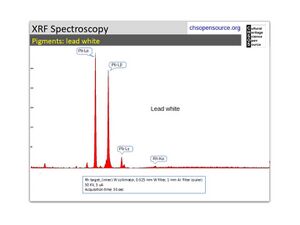
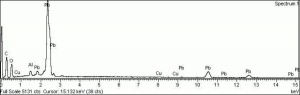
| − | [[[SliderGallery rightalign|Lead White, Oil.TIF~FTIR (MFA)|Lead white (Forbes MFA 519), 50X, 785 nm copy.tif~Raman (MFA)|PIG523.jpg~XRD|f523sem.jpg~SEM|f523edsbw.jpg~EDS|lead white.jpg~Chemical structure]]] | + | [[[SliderGallery rightalign|Lead White, Oil.TIF~FTIR (MFA)|Lead white (Forbes MFA 519), 50X, 785 nm copy.tif~Raman (MFA)|PIG523.jpg~XRD|CHSOS XRF of Lead white.jpg~XRF (CHSOS)|f523sem.jpg~SEM|f523edsbw.jpg~EDS|lead white.jpg~Chemical structure]]] |
==Physical and Chemical Properties== | ==Physical and Chemical Properties== | ||
| Line 51: | Line 50: | ||
* Ashok Roy, Contributed information, November 2007. | * Ashok Roy, Contributed information, November 2007. | ||
| + | * Wikipedia: [https://en.wikipedia.org/wiki/Lead_white Lead white] Accessed April 2024 | ||
* R.J.Gettens, H. Kuhn, and W.T. Chase, "Lead White", ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. ref. index = 1.94; 2.09 | * R.J.Gettens, H. Kuhn, and W.T. Chase, "Lead White", ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. ref. index = 1.94; 2.09 | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 6.70 and ref.index.= 1.94; 2.09 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 6.70 and ref.index.= 1.94; 2.09 | ||
Latest revision as of 13:31, 10 April 2024
Description
A white pigment composed of basic lead carbonate. Lead white (lead hydroxycarbonate), contains about 70% lead carbonate and 30% lead hydroxide chemically combined. Basic lead carbonate occurs in nature as the mineral hydrocerussite, but the mineral form was not commonly used as a pigment; instead lead white has been prepared synthetically from Classical antiquity by exposing metallic Lead to Vinegar (Acetic acid) vapor. Lead acetate forms first, which then reacts with Carbon dioxide from the atmosphere (or from another source). Lead white is a dense, opaque pigment that was mainly used in drying oils where it acts as a siccative. It has also been found in Egg tempera, Glue tempera, and Gum tempera, but it was not considered suitable for Buon fresco techniques. Lead white was the principal white pigment used in paintings and ceramic glazes from ancient times until the early 20th century when it was replaced by Zinc white and Titanium white. Unfortunately, lead white was still found in some exterior house paints and ceramic glazes till the middle of the mid 20th century. In the United States, its use in interior paints was restricted in the 1950s and prohibited in 1978. Lead white can yellow or blacken in the presence of sulfides or alkalis. Additionally, it can be oxidized by microorganisms to form brownish-black lead dioxide. Cremnitz white is a very white, dense type of white lead prepared from Litharge and acetic acid. Occasionally the name lead white has also been used for basic lead sulfate and basic lead silicate.
Synonyms and Related Terms
basic lead carbonate; Pigment White 1; CI 77597; hydrocerussite (mineral); plomo blanco (Esp.); céruse (Fr.); blanc de plomb (Fr.); blanc d'argent (Fr.); Bleiweiss (Deut.); Kremserweiss (Deut.); leyko toy molybdoy (Gr.); psimythio (Gr.); ydrokeroyssitis (Gr.)bianco di piombo (It.); biacca (It.); cerussa (It.); loodwit (Ned.); branco de chumbo (Port.); enpaku (Jap.); bai (Chin.); Flemish white; Vienna white; Berlin white; slate white; Roman white; flake white; Cremnitz white; white lead; London white; silver white; Krems white; Dutch white lead
Physical and Chemical Properties
Dissolves in acids, giving off carbon dioxide. Insoluble in water and ethanol. Fluoresces reddish purple. Pigment has fine, fairly uniform, rounded tabular particles. Converts to yellow lead oxide (massicot) with moderate heat and to red lead oxide at higher temperatures.
| Composition | 2PbCO3.Pb(OH)2 |
|---|---|
| CAS | 1319-46-6 |
| Density | 6.70-6.86 g/ml |
| Molecular Weight | mol. wt. = 775.62 |
| Refractive Index | e=1.94; w=2.09 |
Risks
- Toxic by inhalation or ingestion.
- Skin contact may cause irritation or ulcers.
- Carcinogen, teratogen, suspected mutagen.
- It's use in interior house paints in the U.S. was prohibited in 1978.
- Darkens with exposure to sulfur and alkalis.
- Susceptible to biological degradation.
- Natural Pigments: SDS
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Ashok Roy, Contributed information, November 2007.
- Wikipedia: Lead white Accessed April 2024
- R.J.Gettens, H. Kuhn, and W.T. Chase, "Lead White", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. ref. index = 1.94; 2.09
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 6.70 and ref.index.= 1.94; 2.09
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigments'
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Pigments Through the Ages - https://www.webexhibits.org/pigments/indiv/overview/leadwhite.html - Uniaxial (-), e = 1.94, w = 2.09
- R. Newman, E. Farrell, 'House Paint Pigments', Paint in America , R. Moss ed., Preservation Press, New York City, 1994
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', Japanese Woodblock Prints, Allen Memorial Art Museum, Oberlin College, Oberlin, 1984
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- MSDS Sheet Comment: Baker MSDS Spec gravity = 6.14
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
Record content reviewed by EU-Artech, November 2007.