Difference between revisions of "Limestone"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 1: | Line 1: | ||
| − | [[File:13.4352-SC66701.jpg|thumb|]] | + | [[File:13.4352-SC66701.jpg|thumb|Mastaba chapel wall<br>MFA# 13.4352]] |
== Description == | == Description == | ||
A fine-grain, sedimentary rock. Limestone is composed primarily of [[calcium carbonate]] in the form of [[calcite]] or [[aragonite]]. Trace amounts of [[dolomite]], [[ferric oxide|iron oxide]], [[quartz]], [[clay]], or organic particles can also be present. Due to these impurities, limestone can vary in color from a cream to yellow to pink to brown to dark gray. Limestone is formed from compressed and cemented [[seashell|seashells]] and marine animal skeletons (reefs) or reprecipitation (stalactites, stalagmites). It is softer and more easily worked than marble. [[Chalk]] is a soft, porous, fine-grain limestone. [[Coquina]] is a porous, soft limestone made up of visible shell fragments. Limestone is used as a building stone and for sculpture as well as in the manufacture of [[lime]], [[carbon dioxide]], and [[cement]]. | A fine-grain, sedimentary rock. Limestone is composed primarily of [[calcium carbonate]] in the form of [[calcite]] or [[aragonite]]. Trace amounts of [[dolomite]], [[ferric oxide|iron oxide]], [[quartz]], [[clay]], or organic particles can also be present. Due to these impurities, limestone can vary in color from a cream to yellow to pink to brown to dark gray. Limestone is formed from compressed and cemented [[seashell|seashells]] and marine animal skeletons (reefs) or reprecipitation (stalactites, stalagmites). It is softer and more easily worked than marble. [[Chalk]] is a soft, porous, fine-grain limestone. [[Coquina]] is a porous, soft limestone made up of visible shell fragments. Limestone is used as a building stone and for sculpture as well as in the manufacture of [[lime]], [[carbon dioxide]], and [[cement]]. | ||
| − | [[File:27.1132-E2000CR-d1.jpg|thumb|]] | + | [[File:27.1132-E2000CR-d1.jpg|thumb|Lintel of Kameni<br>MFA# 27.1132]] |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 11: | Line 11: | ||
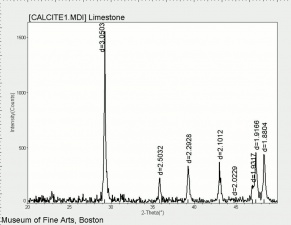
[[[SliderGallery rightalign|CALCITE1.jpg~XRD]]] | [[[SliderGallery rightalign|CALCITE1.jpg~XRD]]] | ||
| + | == Risks == | ||
| − | == | + | No significant hazard. Noncombustible. |
| + | == Physical and Chemical Properties == | ||
Soluble in acids with the evolution of carbon dioxide gas. | Soluble in acids with the evolution of carbon dioxide gas. | ||
| Line 31: | Line 33: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * B.Aston, J.Harrell, I.Shaw, "Stone" in ''Ancient Egyptian Materials and Technology'', P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 40-43. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "limestone" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "limestone" [Accessed January 22, 2002 |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: limestones density = 1.164 - 2.483; calcite density = 2.71 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: limestones density = 1.164 - 2.483; calcite density = 2.71 | ||
| Line 59: | Line 55: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * | + | * Marble Institute: [http://www.marble-institute.com http://www.marble-institute.com] |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.68-2.76 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.68-2.76 | ||
Revision as of 07:47, 28 August 2020
Description
A fine-grain, sedimentary rock. Limestone is composed primarily of Calcium carbonate in the form of Calcite or Aragonite. Trace amounts of Dolomite, iron oxide, Quartz, Clay, or organic particles can also be present. Due to these impurities, limestone can vary in color from a cream to yellow to pink to brown to dark gray. Limestone is formed from compressed and cemented seashells and marine animal skeletons (reefs) or reprecipitation (stalactites, stalagmites). It is softer and more easily worked than marble. Chalk is a soft, porous, fine-grain limestone. Coquina is a porous, soft limestone made up of visible shell fragments. Limestone is used as a building stone and for sculpture as well as in the manufacture of Lime, Carbon dioxide, and Cement.
Synonyms and Related Terms
quina; caliza (Esp.); calcaire (Fr.); calcário (Port.); Kalkstein (Deut.); kalksteen (Ned.)
Risks
No significant hazard. Noncombustible.
Physical and Chemical Properties
Soluble in acids with the evolution of carbon dioxide gas.
| Composition | CaCO3 |
|---|---|
| CAS | 1317-65-3 |
| Mohs Hardness | 3.0 - 4.0 |
| Density | 2.1-2.7 |
Resources and Citations
- B.Aston, J.Harrell, I.Shaw, "Stone" in Ancient Egyptian Materials and Technology, P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 40-43.
- Encyclopedia Britannica, http://www.britannica.com Comment: "limestone" [Accessed January 22, 2002
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: limestones density = 1.164 - 2.483; calcite density = 2.71
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas C. Jester (ed.), Twentieth-Century Building Materials, McGraw-Hill Companies, Washington DC, 1995
- Ancient Egyptian Materials and Technologies, Paul Nicholson, Ian Shaw (eds.), Cambridge University Press, Cambridge, 2000 Comment: B.Aston, J.Harrell, I.Shaw, "Stone"
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Marble Institute: http://www.marble-institute.com
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.68-2.76