Difference between revisions of "Litharge"
| Line 1: | Line 1: | ||
[[File:284 litharge.jpg|thumb|Litharge]] | [[File:284 litharge.jpg|thumb|Litharge]] | ||
== Description == | == Description == | ||
| − | + | [[File:litharge2 C100x.jpg|thumb|litharge at 100x (visible light left; UV light right)]] | |
A heavy yellow powder composed of [[lead monoxide]]. Litharge is prepared as the oxidized product of molten lead that has been stirred or atomized to incorporate air then cooled and ground to form the yellow powder. [[Massicot]], another crystalline form of lead monoxide, occurs naturally but can also be made by heating [[lead carbonate, basic|lead carbonate]] to 300C. Litharge is lightly more orange than massicot due to some formation of [[red lead|red lead oxide]]. Both forms of lead monoxide has been used as a [[drier]] in [[oil]] and as a low-fire [[flux] in making [[ceramic|ceramics]] and [[glass]]. They were used as a yellow pigments in [[paint|paints]] and [[glaze|glazes]]. Thin layers of lead monoxide are used to produce iridescent colors on [[brass]] and [[bronze]]. It has also been used as a filler for [[rubber|rubber]] and to produce artificial [[tortoiseshell]] and [[horn]]. Litharge is mixed with [[glycerol]] to make [[litharge cement|plumber's cement]]. | A heavy yellow powder composed of [[lead monoxide]]. Litharge is prepared as the oxidized product of molten lead that has been stirred or atomized to incorporate air then cooled and ground to form the yellow powder. [[Massicot]], another crystalline form of lead monoxide, occurs naturally but can also be made by heating [[lead carbonate, basic|lead carbonate]] to 300C. Litharge is lightly more orange than massicot due to some formation of [[red lead|red lead oxide]]. Both forms of lead monoxide has been used as a [[drier]] in [[oil]] and as a low-fire [[flux] in making [[ceramic|ceramics]] and [[glass]]. They were used as a yellow pigments in [[paint|paints]] and [[glaze|glazes]]. Thin layers of lead monoxide are used to produce iridescent colors on [[brass]] and [[bronze]]. It has also been used as a filler for [[rubber|rubber]] and to produce artificial [[tortoiseshell]] and [[horn]]. Litharge is mixed with [[glycerol]] to make [[litharge cement|plumber's cement]]. | ||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 12: | Line 10: | ||
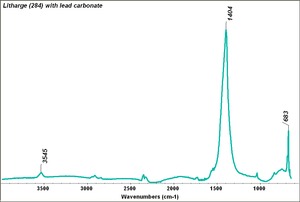
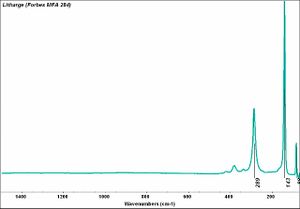
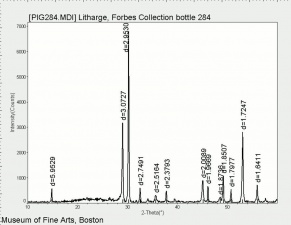
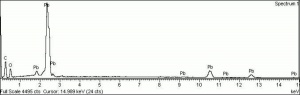
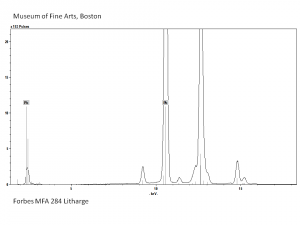
[[[SliderGallery rightalign|Litharge (284).TIF~FTIR (MFA)|Litharge (Forbes MFA 284) (640x445).jpg~Raman (MFA)|PIG284.jpg~XRD|f284sem.jpg~SEM|f284edsbw.jpg~EDS|Slide30 FC284.PNG~XRF]]] | [[[SliderGallery rightalign|Litharge (284).TIF~FTIR (MFA)|Litharge (Forbes MFA 284) (640x445).jpg~Raman (MFA)|PIG284.jpg~XRD|f284sem.jpg~SEM|f284edsbw.jpg~EDS|Slide30 FC284.PNG~XRF]]] | ||
| − | == | + | == Risks == |
| − | |||
| − | |||
| − | ThermoFisher: [https://www.fishersci.com/shop/msdsproxy?productName=AC315855000&productDescription=LEAD%28II%29+OXIDE+POWDER+%3C1+500G&catNo=AC315855000&vendorId=VN00032119&storeId=10652 SDS] | + | * Toxic by inhalation or ingestion. |
| + | * Skin contact may cause irritation or ulcers. | ||
| + | * Carcinogen, teratogen, suspected mutagen. | ||
| + | * ThermoFisher: [https://www.fishersci.com/shop/msdsproxy?productName=AC315855000&productDescription=LEAD%28II%29+OXIDE+POWDER+%3C1+500G&catNo=AC315855000&vendorId=VN00032119&storeId=10652 SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in acetic acid, dilute nitric acid and alkalis. Insoluble in water and ethanol. Turns gray on exposure to sulfur fumes. | Soluble in acetic acid, dilute nitric acid and alkalis. Insoluble in water and ethanol. Turns gray on exposure to sulfur fumes. | ||
| Line 35: | Line 34: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 888 | + | | 888 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 9.40-9.53 | + | | 9.40-9.53 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 52: | Line 51: | ||
File:Litharge, squarish particle at extinction, PPL 1000x.jpg|Litharge particle with squarish morphology at extinction, XPL 1000X | File:Litharge, squarish particle at extinction, PPL 1000x.jpg|Litharge particle with squarish morphology at extinction, XPL 1000X | ||
File:Litharge, squarish particle at brightest point, PPL 1000x.jpg|Litharge particle with squarish morphology at brightest point, XPL 1000X | File:Litharge, squarish particle at brightest point, PPL 1000x.jpg|Litharge particle with squarish morphology at brightest point, XPL 1000X | ||
| − | |||
</gallery> | </gallery> | ||
Revision as of 10:56, 16 September 2022
Description
A heavy yellow powder composed of Lead monoxide. Litharge is prepared as the oxidized product of molten lead that has been stirred or atomized to incorporate air then cooled and ground to form the yellow powder. Massicot, another crystalline form of lead monoxide, occurs naturally but can also be made by heating lead carbonate to 300C. Litharge is lightly more orange than massicot due to some formation of red lead oxide. Both forms of lead monoxide has been used as a Drier in Oil and as a low-fire [[flux] in making ceramics and Glass. They were used as a yellow pigments in paints and glazes. Thin layers of lead monoxide are used to produce iridescent colors on Brass and Bronze. It has also been used as a filler for Rubber and to produce artificial Tortoiseshell and Horn. Litharge is mixed with Glycerol to make plumber's cement.
Synonyms and Related Terms
massicot; lead monoxide; lithargyros (Gr.); plumbum ustum (Latin); Lithargit (Deut.); Massicot (Deut.); litharge (Fr.); litargirio (Esp.); litargirio (giallo di piombo) (It.); massicot (Ned.); litharge (Ned.); litargírio (Port.); yellow lead oxide; plumbous oxide
Risks
- Toxic by inhalation or ingestion.
- Skin contact may cause irritation or ulcers.
- Carcinogen, teratogen, suspected mutagen.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in acetic acid, dilute nitric acid and alkalis. Insoluble in water and ethanol. Turns gray on exposure to sulfur fumes.
In transmitted PPL, the tetragonal alpha-PbO (the beta-PbO referring to massicot) form translucent particles ranging in color from pale yellow and pale yellow-orange to pale green. Some semi-opaque particles of dark reddish-brown color are also reported. Most particles are rounded in shape and other reported morphologies include square, rectangular and acicular shapes. Particles tend to agglomerate into clumps. Relief is very high and RI is greater than 1.662.
In transmitted XPL, the particles are highly birefringent with third-order jewel tones. Extinction is straight, but clumps of particles will not show complete extinction and appear to 'twinkle' as the stage is rotated. Clumps of particles display a strong yellow body color, and smaller particles may exhibit a dull yellowish outline at extinction. Acicular particles are length-fast.
| Composition | PbO |
|---|---|
| CAS | 1317-36-8 |
| Melting Point | 888 C |
| Density | 9.40-9.53 g/ml |
| Refractive Index | 2.51; 2.71; 2.61 |
Additional Images
Resources and Citations
- Eastaugh, N., et. al. "The Pigment Compendium: a Dictionary and Optical Microscopy of Historical Pigments", Butterworth-Heinemann, London, 2008
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density 9.40 and ref. index 2.51; 2.71; 2.61
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996