Difference between revisions of "Thymol"
(username removed) |
|||
| (5 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White, strong smelling crystals. Thymol was first isolated by Neumann in 1719. It occurs naturally in [ | + | White, strong smelling crystals. Thymol was first isolated by Neumann in 1719. It occurs naturally in [[ajowan%20oil|ajowan oil]], horsemint oil, [[eucalyptus%20oil|eucalyptus oil]], and as an extract from thyme plants. Thymol is an [[disinfectant|disinfectant]] and [[fungicide|fungicide]] that has been used to prevent [[mold%20%28fungus%29|mold]] and [[mildew|mildew]] in [[tempera|tempera paint]], [[gesso|gesso]], [[leather|leather]], [[fur|furs]], [[paper|paper]], and [[parchment|parchment]]. It has also been used as a fumigant by heating the crystals. in a sealed cabinet with a low-wattage [[incandescent%20lamp|incandescent lightbulb]]. Thymol is no longer recommended for use because it dissolves [[oil%20paint|oil paint]], [[varnish|varnishes]], and some printing inks. It yellows with age and may discolor or [[tarnish|tarnish]] photographs. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
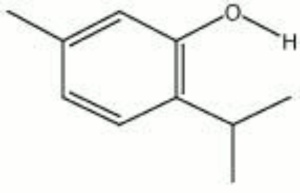
[[[SliderGallery rightalign|thymol.jpg~Chemical structure]]] | [[[SliderGallery rightalign|thymol.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Toxic by inhalation or ingestion. LD50 = 980 mg/kg. | ||
| + | * Skin contact can cause irritation. | ||
| + | * Combustible. | ||
| + | * Thymol dissolves oil paint, varnishes, and some printing inks. | ||
| + | * May stain photographs and discolor paper. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC150330025&productDescription=THYMOL%2C+99%25+2.5KG&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in ethanol, carbon disulfide, chloroform, glacial acetic acid, ether. Slightly soluble in water, glycerol. | Soluble in ethanol, carbon disulfide, chloroform, glacial acetic acid, ether. Slightly soluble in water, glycerol. | ||
| Line 22: | Line 31: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 48-51 | + | | 48-51 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.970-0.979 | + | | 0.970-0.979 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 43: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 233 | + | | 233 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | V.Daniels, B.Boyd, "The Yellowing of Thymol in the Display of Prints" ''Studies in Conservation'' 31:156-158, 1986. | + | * V.Daniels, B.Boyd, "The Yellowing of Thymol in the Display of Prints" ''Studies in Conservation'' 31:156-158, 1986. |
| − | + | * L. Goldberg, A History Of Pest Control Measures In The Anthropology Collections, National Museum Of Natural History, Smithsonian Institution, ''JAIC'' (35):23-43, 1996 | |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 22 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9540 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9540 | ||
| − | * | + | * Marjorie Shelley, ''The Care and Handling of Art Objects'', The Metropolitan Museum, New York, 1987 |
| − | * | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * George Savage, ''Art and Antique Restorer's Handbook'', Rockliff Publishing Corp, London, 1954 |
| − | * | + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 |
| − | * | + | * G.Caneva, M.P.Nugari, O.Salvadori, ''Biology in the Conservation of Works of Art'', ICCROM, Rome, 1991 |
| − | * | + | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 |
| − | * | + | * Marie Svoboda, Conservation Survey Index, unpublished, 1997 |
| − | * | + | * Teri Hensick, contributed information, 1998 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:25, 8 June 2022
Description
White, strong smelling crystals. Thymol was first isolated by Neumann in 1719. It occurs naturally in Ajowan oil, horsemint oil, Eucalyptus oil, and as an extract from thyme plants. Thymol is an Disinfectant and Fungicide that has been used to prevent mold and Mildew in tempera paint, Gesso, Leather, furs, Paper, and Parchment. It has also been used as a fumigant by heating the crystals. in a sealed cabinet with a low-wattage incandescent lightbulb. Thymol is no longer recommended for use because it dissolves Oil paint, varnishes, and some printing inks. It yellows with age and may discolor or Tarnish photographs.
Synonyms and Related Terms
isopropyl-m-cresol; 5-methyl-2-(1-methylethyl)phenol; m-thymol; thyme camphor; thymic acid; 3-hydroxy-1-methyl-4-isopropyl benzene; isopropyl-cresol; 2-isopropyl-5-methyl phenol; 3-hydroxy-p-cymene; timol (Esp.)
Risks
- Toxic by inhalation or ingestion. LD50 = 980 mg/kg.
- Skin contact can cause irritation.
- Combustible.
- Thymol dissolves oil paint, varnishes, and some printing inks.
- May stain photographs and discolor paper.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in ethanol, carbon disulfide, chloroform, glacial acetic acid, ether. Slightly soluble in water, glycerol.
| Composition | (CH3)2CHC6H3(CH3)OH |
|---|---|
| CAS | 89-83-8 |
| Melting Point | 48-51 C |
| Density | 0.970-0.979 g/ml |
| Molecular Weight | mol. wt. = 150.22 |
| Refractive Index | 1.5204 |
| Boiling Point | 233 C |
Resources and Citations
- V.Daniels, B.Boyd, "The Yellowing of Thymol in the Display of Prints" Studies in Conservation 31:156-158, 1986.
- L. Goldberg, A History Of Pest Control Measures In The Anthropology Collections, National Museum Of Natural History, Smithsonian Institution, JAIC (35):23-43, 1996
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 22
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9540
- Marjorie Shelley, The Care and Handling of Art Objects, The Metropolitan Museum, New York, 1987
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- George Savage, Art and Antique Restorer's Handbook, Rockliff Publishing Corp, London, 1954
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- G.Caneva, M.P.Nugari, O.Salvadori, Biology in the Conservation of Works of Art, ICCROM, Rome, 1991
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Marie Svoboda, Conservation Survey Index, unpublished, 1997
- Teri Hensick, contributed information, 1998